Tenvir Tablets
Tenvir Tablets Specification
- Assay
- Not less than 99%
- Grade
- Medicine Grade
- Indication
- Used in combination with other antiretroviral agents for HIV-1 infection and chronic hepatitis B
- Expiration Date
- 24-36 Months from manufacturing date
- Pacakaging (Quantity Per Box)
- 30 Tablets
- Appearance
- White to off-white tablets
- Usage
- Treatment of HIV infection
- Dosage Form
- Tablets
- CAS No
- 147127-20-6
- Storage
- Store below 30C, protect from moisture
- Dosage
- 300 mg
- Molecular Formula
- C9H14N5O4P
- Origin of Medicine
- India
- Salt Composition
- Tenofovir Disoproxil Fumarate
- Medicine Type
- Allopathic
- Shelf Life
- Minimum 2 years
- Packing Type
- Bottle
- Inactive Ingredients
- Lactose, magnesium stearate, microcrystalline cellulose (may vary)
- Marketed By
- Cipla Ltd.
- Strength
- 300 mg per tablet
- Prescription Status
- Prescription Only
- Caution
- To be sold by retail on the prescription of a registered medical practitioner only
- Therapeutic Class
- Antiretroviral agent
- Route of Administration
- Oral
- ATC Code
- J05AF07
About Tenvir Tablets
Tenofovir disoproxil fumarate (a prodrug of tenofovir) which is a fumaric corrosive salt of bis-isopropoxycarbonyloxymethyl ester subordinate of tenofovir. In vivo tenofovir disoproxil fumarate is changed over to tenofovir, a non-cyclic nucleoside phosphonate (nucleotide) simple of adenosine 5-monophosphate. Tenofovir displays movement against HIV-1 switch transcriptase. It is shown in blend with other antiretroviral specialists for the treatment of HIV-1 disease in grown-ups and pediatric patients 2 years old and more established. It is additionally shown for the treatment of endless hepatitis B in grown-ups and pediatric patients 12 years old and more established.
India has numerous producers who make Tenofovir disoproxil. To know more reach us.
3s partnership is Exporter, Supplier, Wholesaler for Tenofovir disoproxil in India.
Additional information :
- Prescribed Dose in Adults and Pediatric Patients 12 Years of Age and Older (35 kg or more)
- For the treatment of HIV-1 or interminable hepatitis B: The measurement is one 300 mg once every day taken orally, without respect to nourishment.
- For patients unfit to swallow tablets, the oral powder detailing (7.5 scoops) might be utilized.
- In the treatment of interminable hepatitis B, the ideal length of treatment is obscure. Wellbeing and adequacy in pediatric patients with constant hepatitis B weighing under 35 kg have not been built up.
Indications :
Get crisis therapeutic help if understanding have any of these indications of a hypersensitive response:
- Hives;
- Trouble relaxing;
- Swelling of your face, lips, tongue, or throat.
- This medicine may cause lactic acidosis (a development of lactic corrosive in the body, which can be lethal). Lactic acidosis can begin gradually and deteriorate over time.Get crisis medicinal help if even gentle manifestations of lactic acidosis occures, for example, muscle agony or shortcoming, numb or cool feeling in your arms and legs, inconvenience breathing, stomach torment, queasiness with spewing, quick or uneven heart rate, dazedness
Purpose and Indications
Tenvir Tablets are indicated for the treatment of HIV-1 infection and chronic hepatitis B, always in conjunction with other antiretroviral agents. Using Tenvir as prescribed by a healthcare professional ensures optimal viral suppression. Appropriate use may contribute to improved immune function and delayed progression of infection-related complications.
Dosage and Administration
Each tablet provides a strength of 300 mg, to be taken orally once daily or as directed by your physician. Its important to swallow the tablet whole, preferably with food, at a consistent time each day. Healthcare professionals will determine individual dosage and duration based on your clinical needs.
Storage and Packaging
Tenvir Tablets are packaged in bottles, each containing 30 tablets. They must be stored below 30C and kept away from moisture to maintain potency. Always check the expiration date, which ranges from 24 to 36 months from the manufacturing date, to ensure their safety and effectiveness.
FAQs of Tenvir Tablets:
Q: How should Tenvir Tablets be taken for optimal effectiveness?
A: Tenvir Tablets should be taken orally once daily, exactly as prescribed by your healthcare provider. Swallow the tablet whole with water, preferably at the same time each day, and ideally with food to enhance absorption. Do not modify your dosage or stop the treatment without consulting your doctor.Q: What is the main benefit of using Tenvir Tablets?
A: Tenvir Tablets, when used in combination with other antiretroviral agents, help control HIV-1 infection and chronic hepatitis B by reducing viral load and preventing disease progression. This promotes a healthier immune system and lowers the likelihood of developing complications.Q: When should Tenvir Tablets not be used?
A: Tenvir Tablets should not be used without a prescription from a registered medical practitioner. Inform your healthcare provider of any allergies or underlying medical conditions. Do not use the medicine beyond its expiration date or if youre allergic to any of its inactive ingredients such as lactose.Q: Where can Tenvir Tablets be purchased?
A: Tenvir Tablets are available through licensed pharmacies in India and internationally, but only with a valid prescription from a registered medical practitioner. They are distributed, supplied, and marketed by Cipla Ltd. and various authorized dealers and wholesalers.Q: What is the process for storing Tenvir Tablets safely?
A: Store Tenvir Tablets below 30C, away from direct sunlight and moisture. Keep the bottle tightly closed and out of reach of children. Always verify the expiration date before use and avoid transferring tablets to other containers to maintain product integrity.Q: What are the possible inactive ingredients in Tenvir Tablets?
A: Tenvir Tablets may contain inactive ingredients such as lactose, magnesium stearate, and microcrystalline cellulose. These ingredients may vary, so consult the packaging or your pharmacist if you have concerns about specific excipients.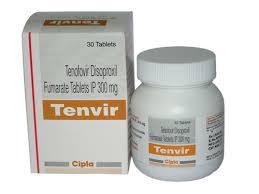

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in HIV AIDS Medicines Category
Ritomune Capsules
Pacakaging (Quantity Per Box) : 60 capsules per box
Indication : Used for the treatment of HIV infection in combination with other medications.
Expiration Date : 24 months from the date of manufacture
Appearance : Offwhite to pale yellow capsules
Usage : Taken orally to help manage and control HIV infections by inhibiting viral replication.
Entecavir Virel Tablets
Pacakaging (Quantity Per Box) : 30 tablets
Indication : Treatment of chronic hepatitis B virus (HBV) infection
Expiration Date : 24 months from the date of manufacture
Appearance : White to offwhite tablets
Usage : Taken orally as directed by a healthcare provider
Nevimune Tablets
Pacakaging (Quantity Per Box) : 30 tablets
Indication : Treatment of HIV1 infection as part of combination therapy
Expiration Date : 2 years from the date of manufacture
Appearance : White to offwhite tablet
Usage : Used in the treatment of HIV/AIDS in adults and children
&
We are accepting bulk order quantity.