Entecavir 0.5 mg Tablets
Entecavir 0.5 mg Tablets Specification
- Pacakaging (Quantity Per Box)
- 30 tablets per box
- Origin of Medicine
- Synthetic
- Expiration Date
- 2 years from the date of manufacture
- Medicine Type
- Antiviral medication
- Usage
- For the treatment of chronic HBV infection in adults with evidence of active viral replication
- Storage
- Store at 20C to 25C (68F to 77F); excursions permitted to 15C30C (59F86F)
- Salt Composition
- Entecavir monohydrate
- Grade
- Pharmaceutical Grade
- Appearance
- White to off-white film-coated tablets
- Dosage
- 0.5 mg per tablet
- Dosage Form
- Tablet
- Assay
- Not less than 98.0% and not more than 102.0% of the labeled amount
- CAS No
- 209216-23-9
- Molecular Formula
- C12H15N5O3
- Indication
- Treatment of chronic Hepatitis B virus infection
About Entecavir 0.5 mg Tablets
Entecavir is an anti-viral drug used for treating chronic hepatitis B infection. It works by preventing the virus from infecting new cells by halting transcription and DNA replication in the multiplication process.FAQs of Entecavir 0.5 mg Tablets:
Q: What is the molecular formula of Entecavir 0.5 mg Tablets?
A: The molecular formula of Entecavir 0.5 mg Tablets is C12H15N5O3.Q: What is the dosage form of Entecavir 0.5 mg Tablets?
A: Entecavir 0.5 mg Tablets are available in a tablet form.Q: What is the storage recommendation for Entecavir 0.5 mg Tablets?
A: Entecavir 0.5 mg Tablets should be stored at 20C to 25C (68F to 77F) with excursions permitted to 15C30C (59F86F).Q: What is the indication for using Entecavir 0.5 mg Tablets?
A: Entecavir 0.5 mg Tablets are indicated for the treatment of chronic Hepatitis B virus infection in adults with evidence of active viral replication.Q: What is the expiration date of Entecavir 0.5 mg Tablets?
A: The expiration date of Entecavir 0.5 mg Tablets is 2 years from the date of manufacture.Q: What is the salt composition of Entecavir 0.5 mg Tablets?
A: The salt composition of Entecavir 0.5 mg Tablets is Entecavir monohydrate.Q: What is the grade of Entecavir 0.5 mg Tablets?
A: Entecavir 0.5 mg Tablets are of pharmaceutical grade with an assay of not less than 98.0% and not more than 102.0% of the labeled amount.
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
More Products in HIV AIDS Medicines Category
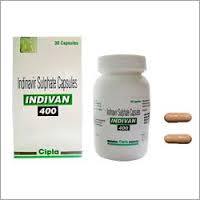
Indivan Capsules
Indication : Used in the treatment of HIV infection as part of combination antiretroviral therapy.
Molecular Formula : C36H47N5O4
CAS No : 123199419
Assay : Not less than 98% purity
Tuvada Tablets
Indication : HIV1 treatment and preexposure prophylaxis (PrEP)
Molecular Formula : C9H14N5O4P (for Tenofovir Disoproxil Fumarate) + Other active ingredients
CAS No : 202138509
Assay : 98% Purity
"We mainly export our products to the foreign countries."
&
We are accepting bulk order quantity.
&
We are accepting bulk order quantity.