Bendamustine Injection
Bendamustine Injection Specification
- Salt Composition
- Bendamustine Hydrochloride
- Indication
- Chronic lymphocytic leukemia, Non-Hodgkin lymphoma
- Dosage Form
- Injection
- Feature
- Lyophilized powder or solution for injection; sterile and pyrogen-free
- Ingredients
- Bendamustine HCl, excipients
- Application
- Intravenous use (IV only)
- Ph Level
- Neutral to slightly acidic (range 6.0-7.5)
- Physical Color/Texture
- Clear, colorless to pale yellow solution
- Fermentation Smell
- Odorless
- Storage Instructions
- Store below 25C; protect from light
- Shelf Life
- 24 months
- Precautions
- Monitor blood counts and liver function
- Reconstitution
- Reconstitute with sterile water for injection
- Dilution Instructions
- Dilute further with 0.9% sodium chloride before administration
- Marketed By
- As per packaging or regulatory source
- Strength
- 100 mg per vial
- Packaging
- Single-use glass vial
- Preservative
- Does not contain preservative
- Contraindications
- Hypersensitivity to Bendamustine or any excipients
- Administration Route
- Intravenous infusion only
- Regulatory Status
- Prescription medicine (Rx only)
About Bendamustine Injection
Bendamustine Injection:-
Bendit (Generic Treanda) is a rationally designed purine analog and alkylator hybrid. It damages the DNA in cancer cells, which leads to the normal path of cell death (apoptosis). It also stops cancer cells from dividing to create new cancer cells.
Bendit (Generic Treanda) is specifically indicated for: 1) the treatment of chronic lymphocytic leukemia where efficacy relative to first line therapies other than chlorambucil has not been established, and 2) indolent B-cell non-Hodgkins lymphoma that has progressed during or within six months of treatment with rituximab or a rituximab-containing regimen.
Bendit (Generic Treanda) is supplied as a powder in a 100 mg vial to be reconstituted with sterile water for intravenous administration.
The recommended initial dose of the drug for the treatment of non-Hodgkin's lymphoma is 120 mg/m2 administered intravenously over 60 minutes on Days 1 and 2 of a 21-day cycle, up to 8 cycles. Bendit (Generic Treanda) administration should be delayed in the event of a Grade 4 hematologic toxicity or clinically significant > Grade 2 non-hematologic toxicity.
Dose modifications for hematologic toxicity: reduce the dose to 90 mg/m2 on Days 1 and 2 of each cycle. If Grade 4 toxicity recurs, reduce the dose to 60 mg/m2 on Days 1 and 2 of each cycle.
Dose modifications for non-hematologic toxicity: reduce the dose to 90 mg/m2 on Days 1 and 2 of each cycle. If Grade 3 or greater toxicity recurs, reduce the dose to 60 mg/m2 on Days 1 and 2 of each cycle.
The recommended initial dose of the drug for the treatment of chronic lymphocytic leukemia is 100 mg/m2 administered intravenously over 30 minutes on Days 1 and 2 of a 28-day cycle, up to 6 cycles. Bendit (Generic Treanda) administration should be delayed in the event of Grade 4 hematologic toxicity or clinically significant >Grade 2 non-hematologic toxicity.
Dose modifications for hematologic toxicity: reduce the dose to 50 mg/m2 on Days 1 and 2 of each cycle; if Grade 3 or greater toxicity recurs, reduce the dose to 25 mg/m2 on Days 1 and 2 of each cycle.
Indications and Dosage
Bendamustine Injection is designed for use in patients with chronic lymphocytic leukemia and non-Hodgkin lymphoma. The specific dosage and administration schedule should be determined by a healthcare professional based on the patients clinical condition and established guidelines. Always adhere strictly to prescribed instructions.
Preparation and Administration
Before administration, the lyophilized powder must be reconstituted with sterile water for injection, then further diluted with 0.9% sodium chloride solution. Administration is by intravenous infusion only. The preparation must be handled under aseptic conditions and used immediately post-reconstitution to ensure sterility, as the vial does not contain preservatives.
Safety and Monitoring
This medication should only be used under the supervision of qualified medical personnel. Blood counts and liver function must be closely monitored throughout the treatment due to potential myelosuppression and hepatic effects. Patients with known hypersensitivity to Bendamustine or its excipients must not use this product.
FAQs of Bendamustine Injection:
Q: How should Bendamustine Injection be prepared for use?
A: Reconstitute each 100 mg vial of Bendamustine with sterile water for injection as detailed in the product insert. After reconstitution, the solution needs to be further diluted with 0.9% sodium chloride before administration by intravenous infusion. This preparation ensures appropriate concentration and safe delivery.Q: What are the main medical conditions treated with Bendamustine Injection?
A: Bendamustine Injection is primarily indicated for the treatment of chronic lymphocytic leukemia and non-Hodgkin lymphoma. It is used under medical supervision and as part of a carefully monitored chemotherapy regimen.Q: When should Bendamustine Injection be administered, and who decides the dosing schedule?
A: The timing and frequency of Bendamustine Injection administration are determined by the treating physician based on the patients condition and specific therapeutic guidelines. All dosing must follow the oncologists protocol to maximize treatment efficacy and minimize risks.Q: Where should Bendamustine Injection be stored prior to use?
A: Store Bendamustine Injection below 25C and protect it from light. Proper storage helps maintain the medications potency and ensures safety up to its 24-month shelf life.Q: What precautions are necessary during Bendamustine Injection therapy?
A: While on Bendamustine therapy, patients should undergo regular monitoring of blood counts and liver function to detect potential adverse effects early. Due to the absence of preservatives, any unused reconstituted solution must not be stored and should be discarded immediately after use.Q: What are the benefits of using Bendamustine Injection in cancer therapy?
A: Bendamustine Injection offers targeted chemotherapy for blood cancers like chronic lymphocytic leukemia and non-Hodgkin lymphoma, aiding in the reduction of tumor cells and potentially improving disease outcomes when used as directed by healthcare professionals.

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Anti Cancer Drugs Category
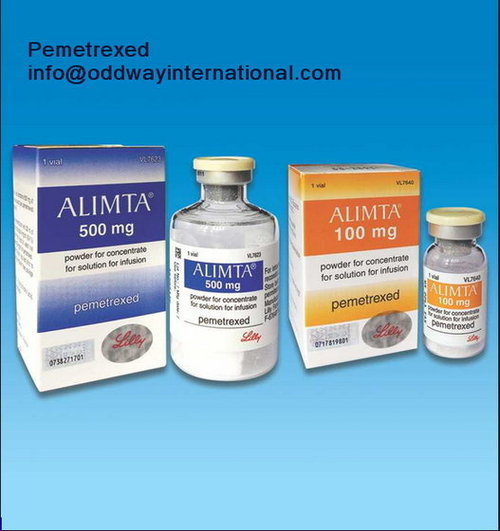
Alimta Pemetrexed 100mg -500mg Injection
Feature : Other, Cytotoxic antineoplastic agent, prescription only
Fermentation Smell : Other, Odorless
Salt Composition : Pemetrexed Disodium 100mg / 500mg per vial
Indication : Malignant pleural mesothelioma, Nonsmall cell lung cancer
Application : Other, For intravenous infusion after reconstitution
Shelf Life : 24 months from the date of manufacture
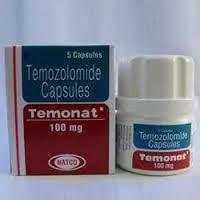
Temonat -Temozolomide 100 mg Tablets
Feature : Other, Alkylating agent, cytotoxic activity
Fermentation Smell : Other, Odorless
Salt Composition : Temozolomide 100 mg
Indication : Treatment of brain tumor, specifically glioblastoma multiforme and anaplastic astrocytoma
Application : Other, Oral administration for chemotherapy
Shelf Life : 24 months from date of manufacture
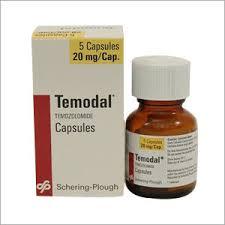
Temodal-Temozolomide Tablets
Feature : Other, Oral chemotherapy agent with proven efficacy in malignant gliomas
Fermentation Smell : Other, Odorless
Salt Composition : Temozolomide
Indication : Used for the treatment of certain types of brain tumours, including glioblastoma multiforme and anaplastic astrocytoma
Application : Other, Anticancer medication
Shelf Life : 2 years
Thalix 100 mg Tablets
Feature : Other, Antiinflammatory and immunomodulatory effect
Fermentation Smell : Other, Odorless
Salt Composition : Thalidomide 100 mg
Indication : Used in treatment of multiple myeloma and erythema nodosum leprosum (ENL)
Application : Other, Oral administration as directed by physician
Shelf Life : 24 months from manufacture
&
We are accepting bulk order quantity.